The FDA has extended the deadline for companies filing a PMA (Premarket Approval Application) for Defibrillator accessories. The new filing deadline will be February 3rd, 2022. This does not affect the previous rule barring companies from marketing Defibrillators that are not on the FDA approved list. This means that Surgery Centers and Hospitals will be able to continue to maintain aging Defibrillators with new batteries and accessories for another year.
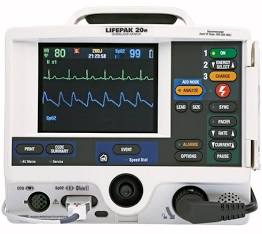
Lifepak 20e 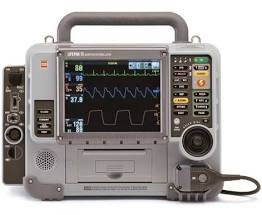
Lifepak 15
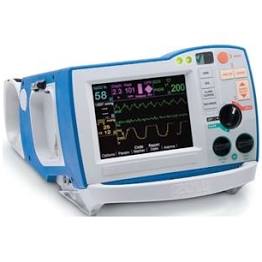
Zoll R Series 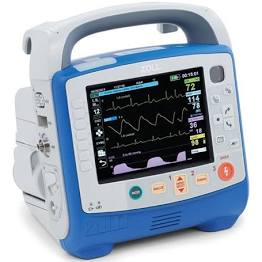
Zoll X Series
